Ways of Working115
Expression Analysis - Agile Market Entry (AME)
Chris Morrison
March 2, 2019
Chris Morrison
March 2, 2019
By Chris Morrison, ViaVerus
Expression Analysis: Expression Analysis (EA) is a specialty CRO (clinical research organization) offering genomic and genetic testing for academic, government, Biotech and pharmaceutical research. At its founding, it provided microarray processing services using the Affymetrix platform. And over its lifetime, it has expanded its genetic and genomic services to included other microarray platforms, sample prep including DNA and RNA isolation, genotyping and RT-PCR.
EA is a pioneer in creating the genomic service provider industry. At the same time, it was pioneering the industry, EA had to build the company and survive the early stages of a nascent industry with limited resources and extreme uncertainty.
Two other pioneers and EA’s two primary competitors were Gene Logic and Paradigm Genetics (later renamed to Icoria). Gene Logic raised over $35million including a $20m Series B and $15m Series C and was sold to Ocimum Biosolutions Ltd for $10million. Paradigm Genetics raised millions in private funds before it went public in a $34.5million IPO in July 2000 before it was sold for $12.5million to Clinical Data in 2005. By contrast, EA raised $4million in two rounds and delivered a healthy return to its investors.
This case study discusses the early days of EA from launch to profitability and the early growth stage. How did EA survive the early days of this nascent industry to become the industry leader and the only early innovator to navigate a successful exit?
Starting a new company is a risky venture. And the more disruptive and innovative the product (or service in EA’s case) and/or business model, the greater the uncertainty and risk associated with going to market. If there is ever an environment that requires an agile framework, this is it. The is exactly the challenge pinpointed by Geoffrey Moore in his iconic publication, “Crossing the Chasm”.
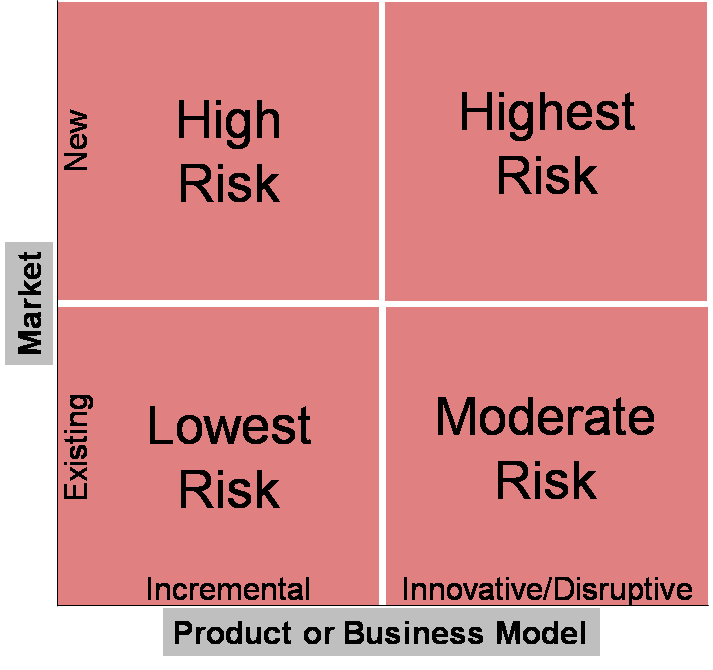
In the case of EA, both the service and business models were novel; genomic services was a nascent industry. Therefore, EA required an agile sales or agile market entry framework to efficiently go-to-market. As evidenced by the life cycle and demise of its competitors, it was a requirement of survival.
Agile market entry (AME) is the time-tested framework for successfully bringing new, disruptive technologies, products and services to market. AME is designed to efficiently navigate the volatile, uncertain and most challenging stage from product launch to scaling revenue. AME is a complete framework for launching innovation: The 4 P’s of AME.
If you follow the thinking of Geoffrey Moore for driving innovation through the Rogers Adoption Curve, AME provides a comprehensive framework to launch a new technology to the Innovators and Early Adopters and lays the path for “crossing the chasm” to the early majority.
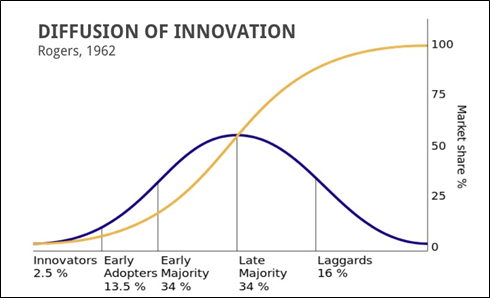
Agile Market Entry is launching innovative, disruptive technologies and efficiently navigating the path from launch to scale while developing the repeatable sales process. Professional sales is executing the proven sales process to scale revenue.
At the time of Expression Analysis’ launch, microarray expression analysis (microarrays) was primarily used as a discovery research tool by academic, government (NIH), pharma and Biotech labs. There were two primary products used in these research labs 1) homebrew slides that were “manufactured” within lab or by commercial vendors and 2) commercial microarray systems with the dominant vendor being Affymetrix. The Affymetrix system generated more consistent and reliable results but at a premium price. At the outset, EA offered a service utilizing the Affymetrix system due to its reliability, growing popularity and market share and the high cost to set up a processing lab.
The initial sales and marketing priority was,
Since EA was a pioneer in building the genomic service provider market, the primary competition was not other service providers but other alternatives for generating genomic data for one’s experiments including,
At launch, sending genomic samples to a commercial service provider (specialty genomic CRO) was not a standard industry practice. If fact, is was uncommon. Building the new market and establishing a new and accepted business model were two major challenges and a high-risk proposition. EA’s sales and marketing team had to be nimble, responsive and creative in order to find, qualify and close new customers. And ultimately turn them into long-term clients which is one of the keystones of a successful service business.
In year one, EA’s early customers turned out to be midsized Biotech companies and disenfranchised researchers in academic and NIH labs that did not have the resources to buy a microarray system or with limited access to a Core Facility. Large Pharma and big Biotech, which were the key source of revenue in the original business plan and where the bulk of research samples resided, had their own facilities and no compelling reason or incentive to outsource. In the early days, our sales team was modifying and iterating the sales and service models as we grew and learned from our customers and market. By remaining lean, nimble and open to changing our strategy and plans, we were able to build a consistent and growing revenue stream, establish market credibility with our early customer base and begin to observe and understand our industry as an insider. Six months post launch, EA closed its first major customer, Johnson and Johnson’s Ortho Clinical Diagnostics (OCD), which immediately became our largest client and brought a new level of credibility.
The other aspect that led to EA’s early success was the close working relationship between the sales and lab teams. One of the unanticipated challenges of our early customer base was many of our clients were just learning how to use genomic data in their research. Often times the samples that we received from a customer were for their first experiment utilizing genomics. In order to have satisfied and happy customers, we needed to evolve our service offerings to meet their unique needs.
Year one revenue came in just shy of $2million which had EA at breakeven. A major accomplishment in a challenging year for a fledgling startup.
If we had operated under the conventional sales model of managing to the business plan with the top-down, command and control management style, we would have been slow to respond, or even worse non-responsive, to our early customer needs. Just as critical, we would have missed key signals from our market pointing to our future and leading to the redesign of our strategy to meet the needs of our customers and market.
And we would have never become a significant player in designing the future of the specialty genomic/genetic CRO industry.
By mid to late year one of operation, we understood the sales and service model for our beachhead customers - academic and NIH labs and small Biotech companies. We understood how to prospect, the value proposition, the sales cycle, the price models, the competitive positioning, the service model for satisfied customers and the repeat business cycles. At the beginning of year two, we hired an Academic and Government Account Manager to continue to develop this market. This freed me up to focus on big Pharma and Biotech – and EA’s future.
EA was founded with the long-term goal of being acquired and delivering a return to the investors. Midway through year one, it was obvious that the beachhead market was insufficient in size or demand to reach a meaningful valuation and exit. EA needed to crack Pharma and Biotech. This is a common situation for startups and new technology launches where the beachhead market generates revenue to build and hopefully sustain the business in the early years but a second market (or more) needs to be entered or created to drive enterprise valuation.
But as we learned post-launch, Pharma and Biotech had internal microarray labs and no compelling reason to outsource. Though Pharma was accustomed to outsourcing clinical trial studies to CROs, there were no GLP labs generating microarray data and for good reason, as no drug submission to the FDA had included microarray data. (GLP or good laboratory practices are the lab standards and procedures required for clinical data.) Genomic data to support clinical trials was the future for EA but there were two critical issues. First, neither drug development companies nor the FDA were ready to include genomic data with new drug submissions and being too early was a real and costly risk. EA wanted to be on the cutting edge but not the bleeding edge. And second, how could a little startup out of RTP, NC compete with the likes of Gene Logic and Paradigm for the Pharma and Biotech business. For the second time in its young existence, EA needed to pioneer a new industry and this time it was going first.
An otherwise small and inconsequential player can have an impact on a large and established industry but it requires a unique position (of value), keen insight and being creative and bold. And often times, it requires thinking beyond the sole interest of the company and to the benefit of the industry as a whole. If the company has the foresight, it can create what I call Fulcrum Market Catalysts. I recognized two such opportunities late in year one.
The first opportunity derived from the general state of the industry. There was a significant amount of variability associated with microarrays and the primary source was associated with sample prep and processing. Lab procedures and quality had a significant impact on sample and data quality and ultimately experiment reproducibility. I recognized this as an opportunity to establish a lab proficiency testing standard to benchmark lab quality. We created EA’s Proficiency Testing Service. In year 2 and 3, EA’s Proficiency Testing Service generated over $145,000 in revenue to the company but more importantly, it evolved into a public/private consortium to standardize reproducibility of microarray experiments across different platforms and labs, the MicroArray Quality Control (MAQC) Consortium. The consortium led by the FDA included other government labs such as the EPA and NIST, all the major microarray platform providers and major academic institutions such as Harvard and UCLA/Cedars-Sinai. And placed EA at the epicentre of the microarray world.
The second opportunity came shortly after attending a workshop between the FDA and the drug development industry to discuss the future of genomics in clinical trials. The use of genomic data was pervasive in industry discovery research and it was just a matter of time before it became part of clinical submissions. The FDA recognized this inevitability and by its own admission, was ill-prepared. Sitting in the workshop, the divide between industry and the FDA was palpable even though both parties were trying to find common ground to prepare for the future. I recognized that EA was in a unique position in that we had no history with the FDA so we had no barriers to working closely; we were a neutral party. And we were experts in an aspect critical to the conversation; every day we were securely transmitting huge data files of genomic data to our clients. Shortly after the DC meeting, I was attending the ABRF meeting in Denver where I happened to meet Dr. Frank Sistare, FDA’s lead in genomics. I had been contemplating a collaboration between the Agency and EA on managing genomic data and when given the chance, immediately took opportunity to mentioned it to Dr. Sistare. He immediately liked the idea and suggested dinner that night. Of course, I accepted. Over dinner, Dr. Sistare, Federico Goodsaid of Schering-Plough (invited by Dr. Sistare) and I created the mock electronic submission of microarray data collaboration, the first ever microarray submission to the FDA.
These two events put EA on the map with Pharma and Biotech. The mock submission led to a press release by the FDA generating immediate market awareness for EA. The MAQC Consortium placed EA in a leadership role for years. We now had our entree into Pharma and Biotech.
At the beginning of year two with EA well established and a new Academic and Government Account Manager in place, I set out to enter the Pharma/Biotech market. In year one I called on the Pharma/Biotech market but only achieved minimal traction. To secure EA’s future, we had to find a way in and the mock electronic submission along with the FDA’s press release cracked open the door.
Now it was time for block and tackle sales. Cold calls, prospecting, schedule meetings and get on a plane to tell our story and get to know our prospects. The press release got me in doors that had previously been closed. We needed to understand Pharma and Biotech’s challenges and were we could help solve their problems. The critical need was GLP data for clinical trials. It was still a bit premature but most companies where now preparing for what was now on the horizon (if not quite here). EA now had the credibility and the demonstrated quality from the Proficiency Testing Service and MAQC Consortium. By the end of year 2, EA had won multiyear contracts with NCI (National Cancer Institute) and the EPA and completed pilots with GlaxoSmithKline, Wyeth and Pfizer with study commitments for year 3 from GSK, Wyeth, Lilly and JNJ.
Expression Analysis (EA) was acquired by Quintiles (now IQVIA) in a private deal in 2012 that delivered an 8x plus return to investors. Up until being acquired, EA was an industry leader. During the same period, the early competitors, Paradigm Genetics and Gene Logic, came and went leaving a path of unhappy investors in its wake. Where Paradigm Genetics and Gene Logic failed, EA launched with an agile, iterative market entry model building a new industry in collaboration with our customers and key players such as the FDA. And to this day, it is still thriving and leading the industry as part of IQVIA’s Q2 Solutions.
– Chris Morrison, Owner of ViaVerus and creator of the Agile Market Entry framework
 Chris Morrison has over 25 years of sales and business development experience bringing products to market. His principal expertise is his ability to formulate and execute strategic marketing and sales plans for new technologies and/or new markets.
Chris Morrison has over 25 years of sales and business development experience bringing products to market. His principal expertise is his ability to formulate and execute strategic marketing and sales plans for new technologies and/or new markets.
Honed over decades of experience, Chris has developed the Agile Market Entry (AME) framework to de-risk new product launches while accelerating the revenue ramp … the true path. AME builds on the concepts of agile and lean while applying proprietary principles and practices to the discipline of the launch sales for innovative and disruptive technologies. It is not just a new sales strategy or tactic … a new twist on conventional sales; AME is a new way of thinking steeped in the iterative, learning concepts of agile delivered with the full body of principles and practices necessary for application and execution.
In his consulting practice, Chris has utilized Agile Market Entry to support all shapes of startups from helping university spinouts come out of the gate to venture-backed commercial startups get their sales back on track.
PHONE 919.304.2760 | EMAIL [email protected]
Please subscribe and become a member to access the entire Business Agility Library without restriction.